Updated February 6, 2026
Vaccinations are the best protection against severe illness and preventable disease for you, your family, and your community. They are safe and effective and can help infants and young children strengthen their immune system. Vaccines in the U.S. are vigorously tested and monitored for safety.
What are Maryland's recommendations for childhood immunizations?
Vaccines remain one of the most powerful public health tools we have to keep ourselves, our families and our communities healthy and safe from disease. Because of vaccines, serious diseases like polio are now distant memories for most Americans. Maryland follows the recommended childhood vaccination schedule distributed by the American Academy of Pediatrics (AAP), last updated February 5, 2026.

Why should my baby or child be vaccinated?
Having babies and young children vaccinated is a very important step in protecting their health. Babies and young children are at an increased risk of acquiring infectious diseases because their immune systems do not have the tools to fight off serious infections and diseases. Early vaccination throughout childhood is needed because it helps create immunity before children are exposed to harmful diseases. Making sure that your family is vaccinated helps to protect the health of your entire community, including those who cannot be vaccinated (children who are too young to be vaccinated, or those who can't receive vaccines for medical reasons). You should talk with your health care provider about any questions you may have about vaccines.
How do vaccines work?
Vaccines work by teaching the body to recognize specific germs, preparing your immune system to fight off future infections. Vaccines induce this immune response without causing the disease or putting the immunized person at risk of the disease's potential complications. For more information, visit the American Academy of Allergy, Asthma & Immunology.
Are vaccines safe? Are all of the ingredients in vaccines safe?
Vaccines in the U.S. are vigorously tested and monitored for safety. Vaccines are some of the safest and most effective medications available, and they have prevented many dangerous childhood illnesses. Vaccines are only given to children after long and careful review by many scientists, doctors, and public health experts. The benefit of the protection that children receive from vaccination outweighs the vast majority of possible side effects that could occur.
The ingredients in vaccines (like inactivated viruses/bacteria, stabilizers, and adjuvants) are also carefully studied. As a result, the formulas have been refined over time. Some of the most commonly debated ingredients are mercury and aluminum. Mercury-containing Thimerosal has been removed from or reduced to trace amounts in nearly all vaccines routinely recommended for children under 6 years old since 2001, with the exception of some formulations of flu vaccines. Aluminum salts are used as adjuvants in some vaccines to help the body build a stronger immune response. The amount of aluminum in vaccines is very small, less than the amount people are exposed to daily through food, water, and breast milk or formula. Aluminum-containing vaccines have a long history of safe use and are well-studied. Learn more from the AAP about Vaccine Safety & Efficacy.
Is it safe to skip or delay vaccinations in infancy until older ages?
The immunization schedule is based on the best timing for each vaccine to provide the most protection when babies are most vulnerable to vaccine-preventable diseases. Developing immune systems are not equipped to fight off these infections, which can quickly become severe, life-threatening, or lead to permanent disability. Following the AAP's schedule ensures they receive protection when the risk of serious complications from a disease is highest, and before the natural protection (antibodies passed in utero or through breastmilk) wears off, preventing unnecessary gaps in immunity.
What are the current vaccine requirements for school-aged children?
Students in public preschool and K-12 schools are subject to the Vaccine Requirements For Children, by Maryland law, available on the Maryland Department of Health Vaccine Requirements webpage. COVID-19 and flu vaccines are not required for students to attend school, but MDH continues to encourage that children stay up to date on all vaccines recommended for them.
Is it safe to get multiple vaccines in one visit?
The recommended childhood immunization schedule often involves giving several vaccines during a single visit (or “simultaneous administration.") Sometimes, vaccines for conditions are combined into a single shot by the manufacturer, which decreases the number of injections given. Simultaneous or combination vaccines are safe, and generally do not cause adverse events at a greater rate than their individual vaccine components. Before approval, a combination vaccine must be carefully tested to ensure it is as safe and effective as each of the individual vaccines given separately. These protocols help families stay on the immunization schedule without additional appointments or more needle sticks than necessary. The entire childhood vaccine schedule exposes a child to fewer antigens (foreign substances that trigger an immune response) than they experience every day through normal activities, like crawling and eating.
Do childhood vaccines cause autism?
No. There is no credible evidence that shows a link between vaccines and autism. Over the years, studies and systematic reviews that include millions of children around the world have consistently found no evidence that vaccines cause autism. You can learn more here: AAP Fact Checked: Vaccines: Safe and Effective, No Link to Autism.
People with autism deserve understanding, respect, inclusion, and support. It does a disservice to them, the progress we have made in understanding autism, and public health as a whole when these types of unsubstantiated claims are made.
Do childhood vaccines cause allergies, eczema or asthma?
No. Vaccines help protect people from serious diseases, especially those with asthma or other allergic conditions. Large, well-controlled epidemiologic studies do not support the claim that vaccines cause allergies or asthma.
Do childhood vaccines cause sudden infant death syndrome (SIDS)?
No. There is no link between vaccines and SIDS, as confirmed by numerous large-scale studies and research reviews over many years. In fact, the incidence of SIDS is the same in children who do and do not receive vaccines. SIDS deaths in the U.S. have actually dropped by more than 50% since the early 1990s, during the same timeframe that infant immunization coverage has increased.
Does Maryland recommend babies get vaccinated for hepatitis B at birth?
Yes. The Maryland Department of Health recommends that all children born in Maryland receive a birth dose of hepatitis B vaccine in accordance with the long-standing evidence-based recommendations of the AAP, and should complete the full vaccination series within 18 months. MDH has issued a Standing Order that authorizes qualified health care professionals to administer the hepatitis B vaccine series using the most current evidence-based guidelines endorsed by the American Academy of Pediatrics as standards, and exercising reasonable clinical judgment in vaccinating their patients.
The hepatitis B vaccine is highly effective in preventing infection in newborns. The vaccine teaches the immune system how to recognize and resist the virus faster than the virus can multiply and cause disease. Babies should get a second dose of hepatitis B vaccine when they are 1 month to 2 months old, and another dose when they are 6 months to 18 months old to maximize lifelong protection. These recommendations align with the American Academy of Pediatrics' Recommended Child and Adolescent Immunization Schedule.
Maryland is a member of the Northeast Public Health Collaborative, which also recently issued a statement of support of the Hep B vaccine birth dose.
Why is it important for babies and children to get vaccinated for hepatitis B?
Hepatitis B is a viral infection that attacks the liver, and can cause acute and/or chronic disease. Infection is spread through blood or body fluid, such as during birth (perinatal transmission) or through close contact with an infected caregiver or household member. Not all people will experience symptoms or know they are infected. Up to 2.4 million people are estimated to have hepatitis B infection in the United States, but only 50% are unaware of their infection.
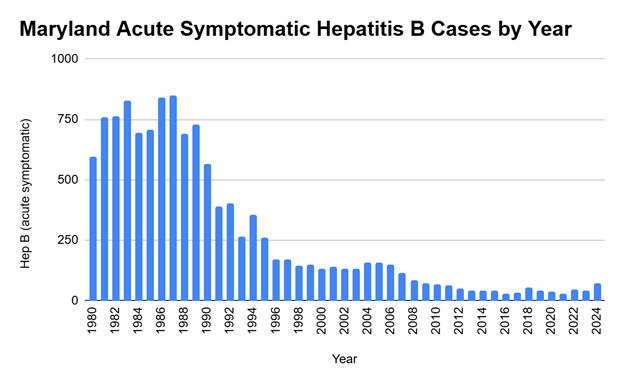
Hepatitis B infection is particularly devastating to infants; 90% of those infected with the virus in the first year of life develop chronic hepatitis B. Chronic infection can lead to liver damage, liver failure, liver cancer, or even death. One in four people infected with hepatitis B virus during childhood dies from liver cirrhosis or liver cancer later in life. The birth dose has led to an overall reduction in hepatitis B transmission, disease, and death in the U.S.
This graph shows the dramatic decrease in acute hepatitis B infections over time, with a notable decline beginning in 1991, when the Centers for Disease Control and Prevention (CDC) recommended universal hepatitis B vaccines for infants beginning at birth.
![]()
Are hepatitis B vaccines safe and effective? Do they have any side effects?
The hepatitis B vaccine has been tested extensively for safety and efficacy. When given as recommended, the hepatitis B vaccine is 80-100% effective in preventing infection or clinical hepatitis in those who receive the complete vaccine series. Hepatitis B vaccine has a strong safety profile. Side effects for newborns and infants are usually mild and resolve within 24-48 hours. No short- or long-term serious adverse events or deaths have been found to be caused by the vaccine. Severe adverse reactions to immunization (such as allergic reactions) are extremely rare, estimated to occur at a rate of one per 1 million doses.
Why give the hepatitis B birth dose to all babies rather than just to those who are at risk?
Since 1991, the Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics have recommended universal hepatitis B vaccination. The goal of a universal approach is to eliminate or drastically reduce a disease for everyone. After adopting this protocol in 1991, hepatitis B infections in children and teens decreased by 99%. The CDC recommendation was reviewed and reinforced in 2002, 2004, 2005 and 2018.
A “risk-based" approach for newborns (testing during prenatal care and only vaccinating babies born to parents with known infection) has not been effective enough in preventing transmission in infancy and early childhood. For example, up to 16% of pregnant people in a 2023 study did not receive the recommended hepatitis B screening that would identify a potential infection.
Is it effective to delay hepatitis B vaccination until later than at birth?
All newborns should receive a hepatitis B vaccine birth dose within 24 hours of delivery. A universal birth dose of hepatitis B vaccine prevents mother-to-infant transmission in 70-90% of cases. It also prevents household transmission between family members and other caregivers in the first months of life. (The hepatitis B virus can live on surfaces such as toothbrushes, washcloths, and nail clippers for a week or more.) Delaying the first dose has no known short- or long-term safety benefit for infants and children. Delaying hepatitis B vaccination does miss a crucial period of potential exposure, putting infants at risk.
Does insurance cover the hepatitis B vaccine?
Yes, in most cases. The actions taken by ACIP on December 5, 2025 should not impact insurance coverage of any hepatitis B vaccine dose in Maryland.
The hepatitis B birth dose is an existing, reimbursable cost within hospital global budgets. For other doses, Maryland law requires regulated insurers to cover the hepatitis B vaccine. Maryland is unable to regulate self-funded health insurance plans, Medicare, Medicare Advantage, Tricare, or Veterans Administration benefits under federal law. However, in September 2025, America's Health Insurance Plans announced a commitment by health plans to continue coverage of vaccines recommended at that time, including hepatitis B, at zero cost-sharing through 2026.
Maryland Medicaid (both the HealthChoice Maryland managed care and fee-for-service programs) covers the vaccine for its participants as indicated by the MDH Childhood Immunization Schedule. As federal vaccine policy evolves, MDH will provide updates about any changes to Maryland Medicaid policies and coverage.
What if I have more questions about vaccines?
Speak with your health care provider about what vaccinations are recommended, the risks and benefits, and/or about vaccine availability.
The American Academy of Pediatrics has many resources for parents and caregivers interested in learning more about immunizations, including at healthychildren.org.
Questions about vaccines for respiratory diseases, like COVID-19, flu and RSV? See our FAQ here.

