May 12, 2021
Deidre McCabe, Director, Office of Communications, 410-767-3536
Charlie Gischlar, Deputy Director, Media Relations, 443-463-7234
Maryland Department of Health Statement on FDA and CDC Support of the Pfizer-BioNTech COVID-19 Vaccine for Adolescents Aged 12 to 15
On Monday, May 10, the Food and Drug Administration (FDA) amended the Emergency Use Authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to expand its use in adolescents 12 to 15 years of age. FDA said in a
statement:
“The FDA has determined that Pfizer-BioNTech COVID-19 vaccine has met the statutory criteria to amend the EUA, and that the known and potential benefits of this vaccine in individuals 12 years of age and older outweigh the known and potential risks, supporting the vaccine’s use in this population.”
Earlier today, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) issued recommendations for the use of the Pfizer-BioNTech vaccine. In officially accepting ACIP’s recommendations, CDC Director Dr. Rochelle Walensky
said:
“For vaccination to do its job, we must do our critical part. That means vaccinating as many people as possible who are eligible. This official CDC action opens vaccination to approximately 17 million adolescents in the United States and strengthens our nation’s efforts to protect even more people from the effects of COVID-19. Getting adolescents vaccinated means their faster return to social activities and can provide parents and caregivers peace of mind knowing their family is protected.”
The Maryland Department of Health (MDH) strongly supports use of the Pfizer-BioNTech vaccine in adolescents 12 to 15 years of age, and encourages providers to make appointments available to this population immediately.
“The Pfizer-BioNTech COVID-19 vaccine has met FDA’s rigorous scientific standards for safety and effectiveness in adolescents aged 12 to 15,” said MDH Deputy Secretary for Public Health Services Dr. Jinlene Chan. “In clinical trials with thousands of participants aged 12 to 15, the Pfizer-BioNTech vaccine demonstrated 100% efficacy against infection and robust antibody responses, and it was well tolerated.”
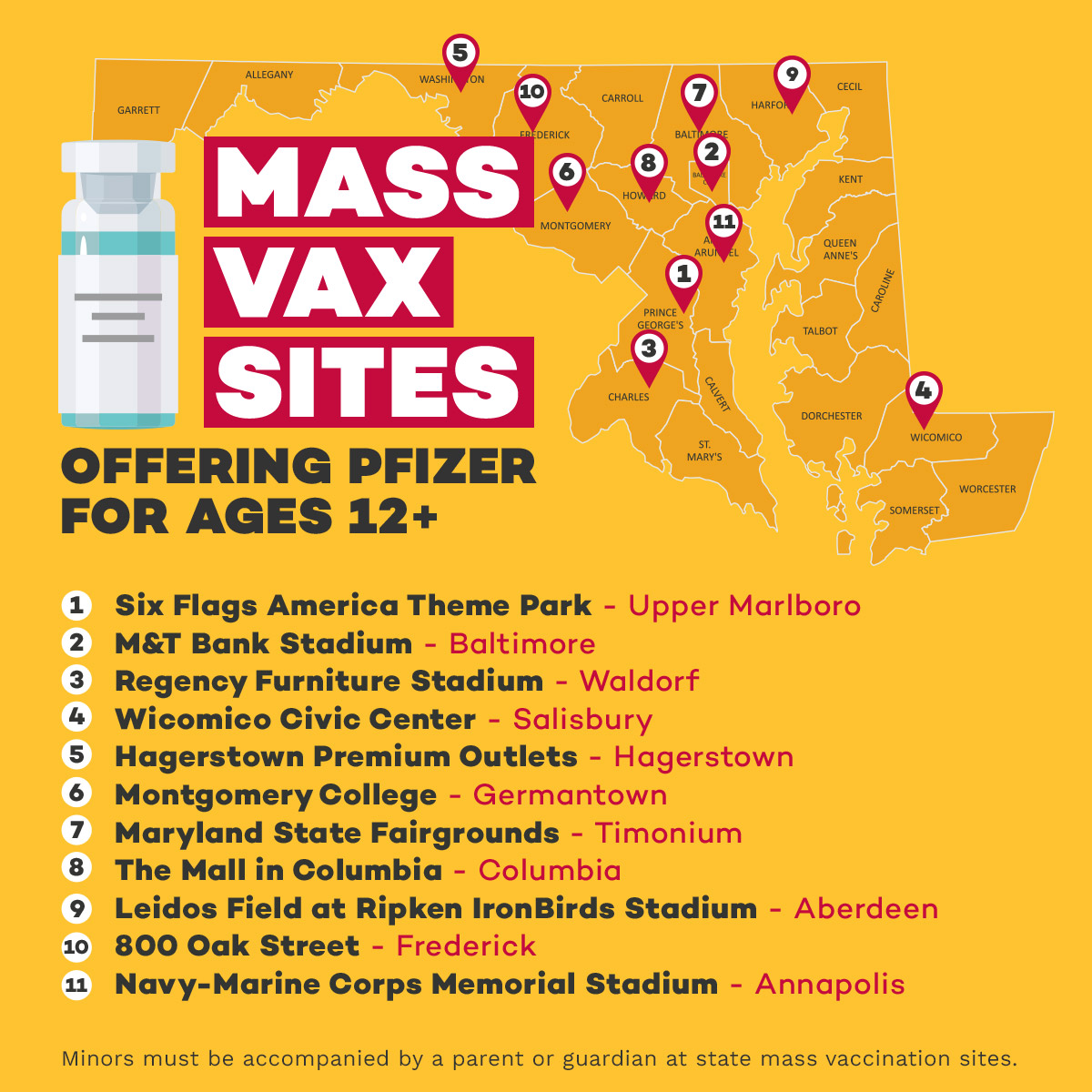
WHERE ADOLESCENTS 12+ CAN GET THE PFIZER-BIONTECH VACCINE
COVID-19 vaccines for adolescents aged 12 to 15 are available currently at the following locations:
- Eleven of Maryland's mass vaccination sites. No appointment is required, although Marylanders can book an appointment to guarantee a time slot.
- 300 pharmacies across the state. Schedule an appointment at the location most convenient for your adolescent.
Visit
COVIDvax.Maryland.gov to find a comprehensive directory and book appointments at sites that are able to accommodate patients aged 12 to 15.
In the coming weeks, COVID-19 vaccines will be available to adolescents aged 12 to 15 in a growing number of locations, to include pediatricians’ offices and other community locations.
“One of our primary goals is to ensure that vaccines are accessible to adolescents through several distribution points,” added Chan. “By working with pediatricians, schools, primary care physicians, and other community providers, we will make it easy for this newly eligible age group to get the vaccines needed to stay safe and help the state recover fully. We encourage families that have questions to contact their child’s healthcare provider.”
Pediatricians and other healthcare providers should carefully review FDA’s fact sheets for
Healthcare Providers Administering the Vaccine (Vaccination Providers) and for
Recipients and Caregivers.
###
The Maryland Department of Health is dedicated to protecting and improving the health and safety of all Marylanders through disease prevention, access to care, quality management and community engagement.

