Purpose
The NUTRIENT SECTION provides analytical and technical services to determine the suitability of drinking water for human consumption and/or effectiveness of wastewater treatment systems. Nitrogen and phosphorus testing help to determine the quality of the Bay waters and support the evaluation of the effectiveness of the nutrient reduction strategies used in the Chesapeake Bay recovery efforts.
The Nutrient Section is one of the two laboratories that make up the Inorganics Analytical Laboratory. Nutrient Section analyzes drinking water, wastewater, groundwater, surface water, sediments, sludges and special project samples for compliance monitoring programs in support of federal and State environmental regulations such as SDWA, CWA, and Chesapeake Bay Program.
Instrumentation
The Nutrient Section uses flow injection analyzers (FIA); Lachat 8000 and 8500 series,
HACH spectrophotometers,and discrete multi-chemistry analyzers.
Discrete Multi-Chemistry Analyzer

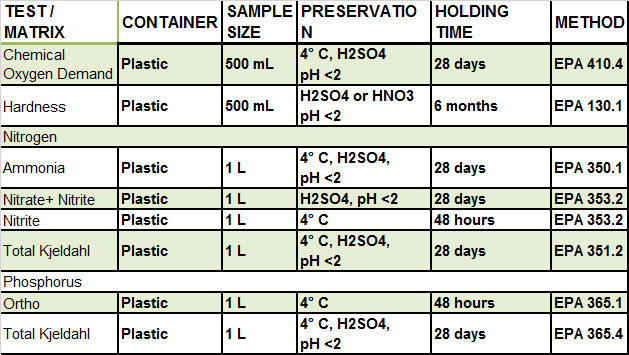
Elements Routinely Analyzed
The Nutrient Section provides analytical and technical services to determine nitrogen compounds (ammonia, nitrate, nitrite and total nitrogen), phosphate and total phosphorous for the suitability of drinking water for human consumption and/or effectiveness of wastewater treatment systems. Nitrogen and phosphorus testing help to determine the quality of the Bay waters and support the evaluation of the effectiveness of the nutrient reduction strategies used in the Chesapeake Bay recovery efforts.
Potential Health and Environmental Concerns
Consumption of water contaminated with a high concentration of nitrate is harmful to small children and pregnant women. High levels of nitrate may cause methemoglobinemia (Blue Baby Syndrome), a condition where the blood is unable to carry enough oxygen to support the cells.
In the environment, excess levels of nitrogen and phosphorus cause algae blooms that block the sunlight, kill the bay grasses and deplete the oxygen in the water when they decompose. This creates “dead zones” where marine life cannot survive

